I am enrolled in a phase 3 randomized clinical trial of the vaccine everyone has forgotten about, the wallflower no one has asked to the prom, the AstraZenenca ChAdOx1 nCoV-19 vaccine against SARS-CoV-2. This vaccine takes the coronavirus' genetic material into a neutralized common cold virus. The modified virus mimics COVID-19, triggering immunity to COVID-19.
Late last spring, the Phase 1/2 trials of about 1000 people included a subgroup of ten patients who got a second dose at 28 days. Phase 1/2 trials ask two questions: is this safe, does it do what we think it will do in actual humans?
91% of the group followed for efficacy ended up with an immune response. 100% of the ten people on the two-dose trial ended up with an immune response. Why are these results not as sexy as the Moderna and Pfizer 94% vaccines? It’s Phase 1/2. It wasn’t very many people. All these findings did was kick-off the Phase 3 trials.
Phase 3 is when they prove it works better than a placebo and show by how much. it also gets the studied drug in the arms of a whole bunch more people, since they basically know at this phase it is worth looking into and doesn’t seem to harm people. My left arm is one of those arms.
I signed up for this study a long time ago, back when sirens blared outside my window twenty-four seven. When they contacted me, I realized that I had assumed I had not been selected. I was wrong.
The screening process involved a lot of reading, and I did a lot more. This was the vaccine whose trial was interrupted over the summer because three subjects ended up with serious illness. One had latent multiple sclerosis, one had transverse myelitis, and one had Guillain-Barrè syndrome, which may or may not have been exacerbated by the vaccine. The trial was resumed after they sorted all that out.
The screening process involved extensive education about all of the above. I signed multiple consent forms that explained all that was known and unknown about the trial, had a thoughtful talk with both the clinician actually working on me and the primary investigator for the study, who stopped in to chat.
They then drew the blood they told me they would take and rammed a swab into my naso-pharynx to have a sample for a covid test. They told me they would hold on to the sample for three days without submitting it to determine if I had covid before getting the vaccine, should we need to know that (i.e., if I get sick).
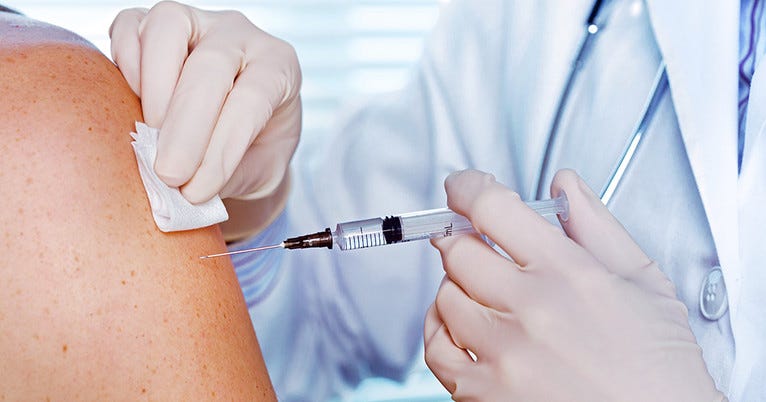
Then came the needle. I was warned that it would hurt. It did not. The first thing that came to mind after they administered the study dose was I got the saline.
Eight hours later I have felt something, a bit of fatigue, but my arm does not hurt at all and I can’t find the vaccination site when I run my hand over the shoulder they put it in. I still think I got the placebo.
That’s okay, control subjects are just as important to what we’re doing here, and I didn’t enroll because I was trying to do anything I could to get a vaccine in me. I enrolled to support vaccine research. I enrolled for you.
They did tell me that if an approved vaccine becomes available to me, and I am in the control group, they will un-blind me and let me know that I can get vaccinated just like I would have been able to without enrolling in the study, should that day arrive.
Otherwise, the study design anticipates unblinding everyone at around six months (it is a two year study) anyway. So, the bottom line is I will know in six months or less if I just got vaccinated. I don’t deny that I hope I did. It would be great to get all the way through this without getting sick.
One interesting twist is that I could, legally, get tested for covid immunity on my own. This would reveal to me immediately what they shot in my arm today. I won’t, but surely someone will, or has. I confess the thought has crossed my mind. I’d like to be covid-immune, or rather know as soon as possible whether I am.
I look forward to going back for the follow-up visits. They were very pleasant people, well-organized and thoughtful. It was in one of the major medical centers in Manhattan, it was also nice to just walk the halls of a hospital again (and not be a patient). I feel at home there. These are my people.
So, there’s a 50% chance I’ve been vaccinated 261 days after I went home from work for the last time in 2020 becuase of a pandemic caused by a completely novel virus humanity first discovered less than a year ago.
These are amazing times.